Introduction
What is a semiconductor?

Materials can be catagorised into conductors, semiconductors
or insulators by their ability to conduct electricity. It is a popular
belief that insulators do not conduct electricity because their valence
electrons are not free to wander throughout the material. In fact they
are free to move around, however, in an insulator there are as many electrons
as there are energy levels for them to occupy. If an electron swaps place
with another electron, no change is made, since individual electrons are
indistinguishable. There are higher energy levels, but to promote the
electrons to such a high energy levels requires an enormous voltage. Metals
conduct electricity easily. In this case, the energy levels between the
conduction and valence band are closely spaced and there are more levels
than electrons so very little energy is required to find new energies
for electrons to occupy. The resisistivity of a material is measure of
how difficult it is for a current to flow. Semiconductors have a resistivity
between 10-4<r>108 W
m, although these are rough limits. The band theory of materials
explains qualitatively the difference between these types of materials.
Electrons belong to the class of particles Fermions that have the property:
only two electrons, each with opposite spin, can occupy a single energy
level. As more electrons are brought together they are forced to occupy
energy levels from the lowest energies upwards. However, some energy levels
are forbidden because of the electrostatic potential of the crystal structure.
 The Kronig-Pennie
model describes a simple quantum mechanical model of this.The allowed
energy levels form bands. In semiconductors, the highest filled level
at T=0 is known as the valence band. Electrons in the valence band do
not participate in the conduction process. The first unfilled level above
the valence band is known as the conduction band. The vacuum level is
defined as the energy required to extract an electron from the conduction
band until it is free from the potential of the crystal structure. In
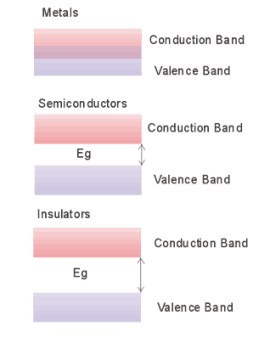
metals, there is no forbidden gap; the conduction band and the valence
band overlap, allowing free electrons to participate in the conduction
process. Insulators have an energy gap that is far greater than the thermal
energy of the electron, while semiconductor materials the energy gap is
typically between1-3eV. The diagram below summarises the energy band model
of materials.
The Kronig-Pennie
model describes a simple quantum mechanical model of this.The allowed
energy levels form bands. In semiconductors, the highest filled level
at T=0 is known as the valence band. Electrons in the valence band do
not participate in the conduction process. The first unfilled level above
the valence band is known as the conduction band. The vacuum level is
defined as the energy required to extract an electron from the conduction
band until it is free from the potential of the crystal structure. In
metals, there is no forbidden gap; the conduction band and the valence
band overlap, allowing free electrons to participate in the conduction
process. Insulators have an energy gap that is far greater than the thermal
energy of the electron, while semiconductor materials the energy gap is
typically between1-3eV. The diagram below summarises the energy band model
of materials.